FMEA.dev
New version (1.0) released, featuring major additions and improvements. Try it! Performing Failure Mode and Effect Analysis (FMEA) can be tedious. This is, in part, due to unhelpful software tools or, rather, inappropriate use of software tools. After all, what problem can’t be solved with a clunky Excel spreadsheet? Above all, though, FMEA brings value, when applied appropriately (and there lies a great deal of subtlety that I won’t go into here). Although there is an abundance of enterprise level, solve-everything reliability tooling, often containing FMEA-specific tools, there is a scarcity of light-weight tools optimized specifically for FMEA, especially those which are web-based and free-to-use.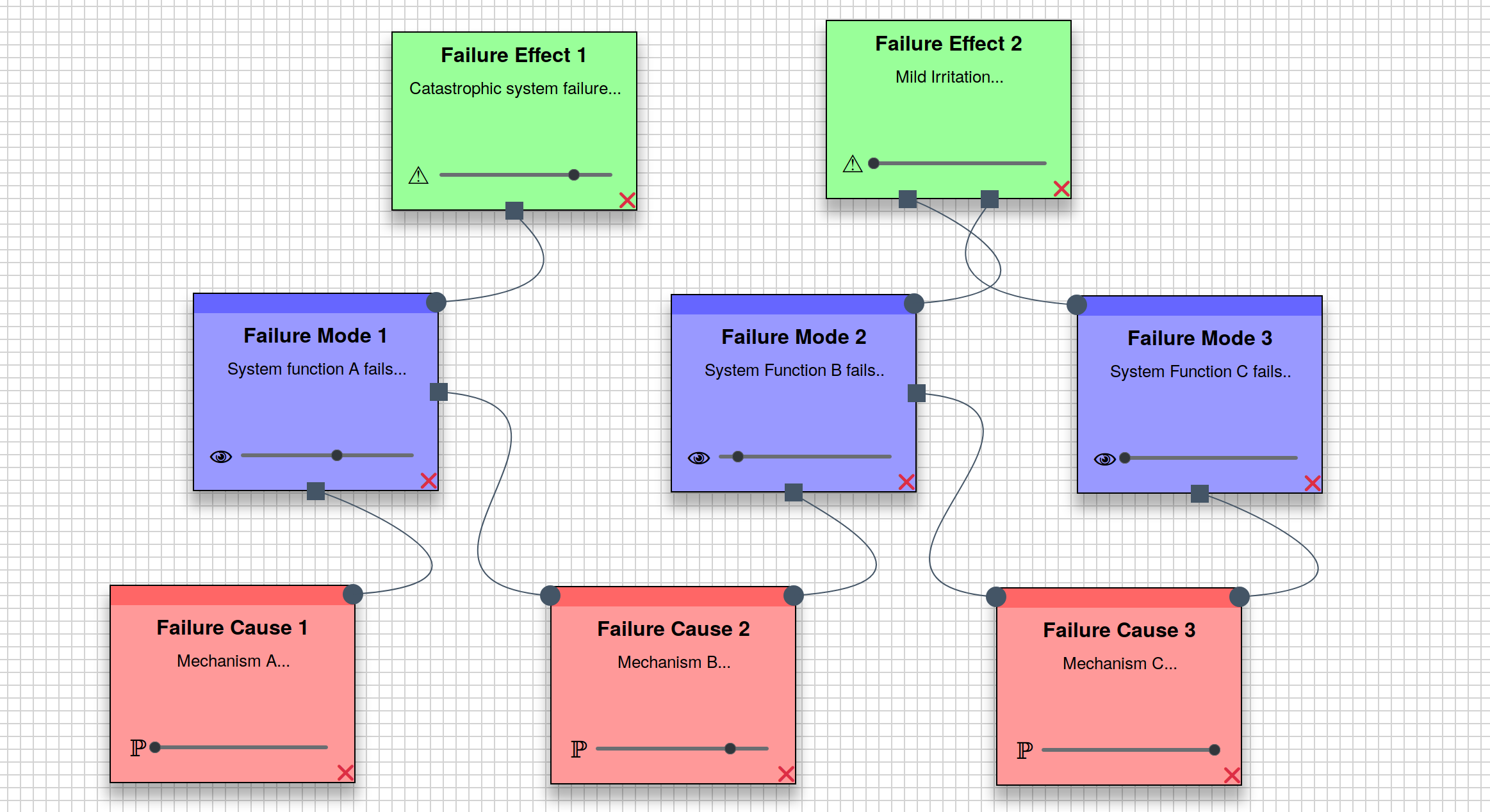
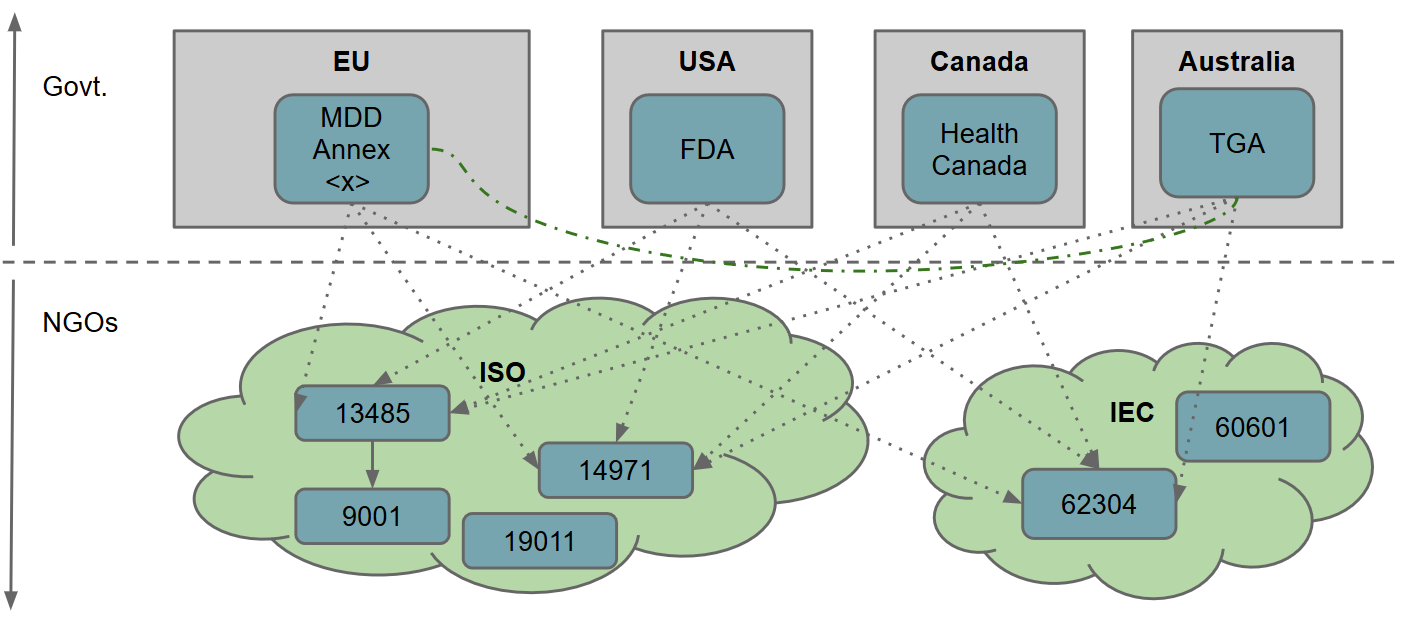
Medical Devices & the MDD
Update: although the content of these slides is still broadly relevant, bear in mind that the MDD is being replaced by the MDR. For a few years I worked in role that doubled as both software engineer and quality assurance officer in a medical device startup. One of the most challenging aspects for small (and large!) companies that are fresh to the market is going through the applicable legislation and subsequent quality assurance (and safety) procedures for releasing a new medical device.