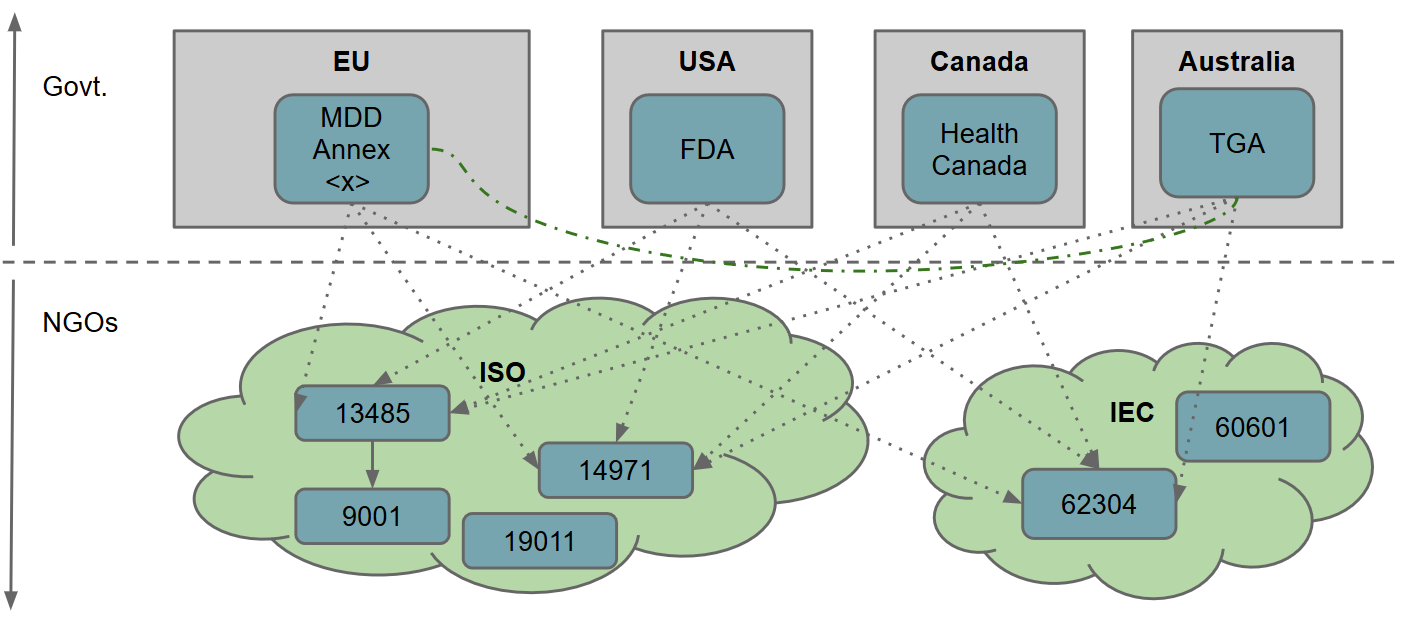
Medical Devices & the MDD
Update: although the content of these slides is still broadly relevant, bear in mind that the MDD is being replaced by the MDR. For a few years I worked in role that doubled as both software engineer and quality assurance officer in a medical device startup. One of the most challenging aspects for small (and large!) companies that are fresh to the market is going through the applicable legislation and subsequent quality assurance (and safety) procedures for releasing a new medical device.