Medical Devices & the MDD
Update: although the content of these slides is still broadly relevant, bear in mind that the MDD is being replaced by the MDR.
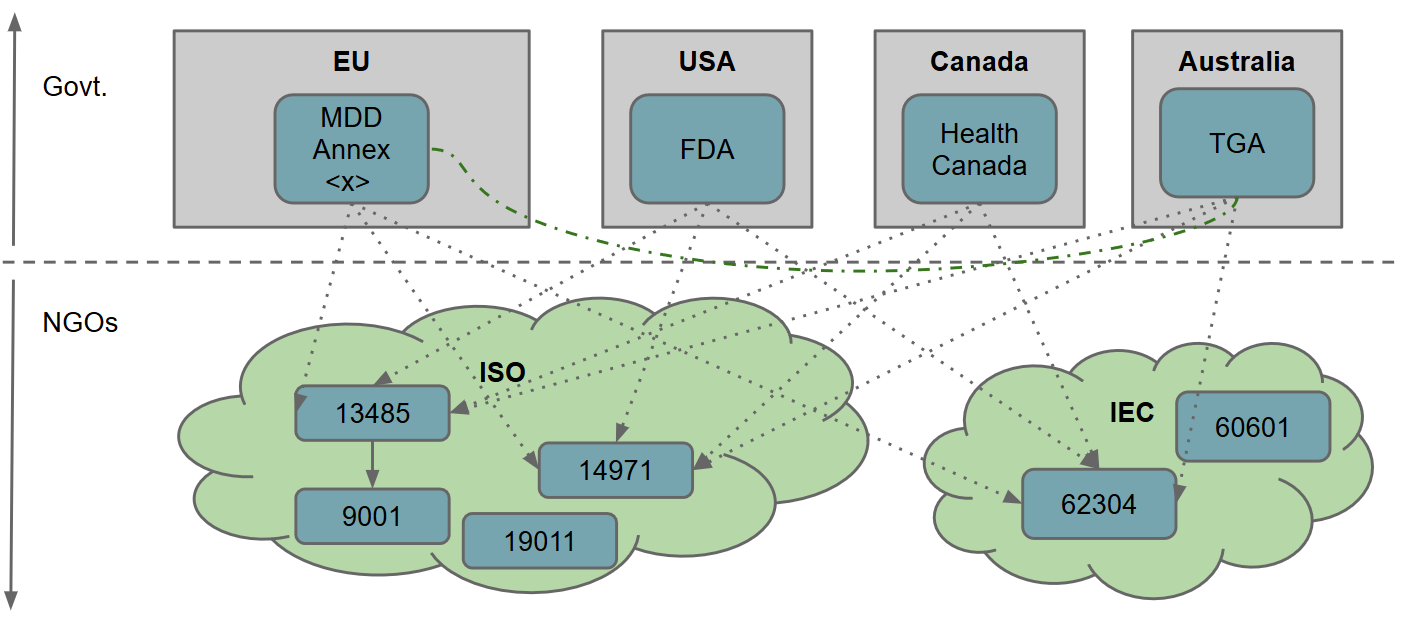
For a few years I worked in role that doubled as both software engineer and quality assurance officer in a medical device startup. One of the most challenging aspects for small (and large!) companies that are fresh to the market is going through the applicable legislation and subsequent quality assurance (and safety) procedures for releasing a new medical device.
During my years in this role I accumulated experience from taking software concepts into marketed hospital medical devices, mostly active within the EU. When I departed this role I tried to give a bird’s-eye-view presentation/workshop of everything that I’d learned so that my colleagues could continue the company’s good work.
You should be able to see the Google Drive presentation embedded above. If not, you can access it directly here.
Nothing in the presentation should be considered as definitive or complete - indeed, professionals working in the area will likely have considerable criticism about the relative weight given to certain areas. For those new to the field, however, it can serve as a helpful insight into an otherwise impenetrable topic.
